updated: August 17, 2012
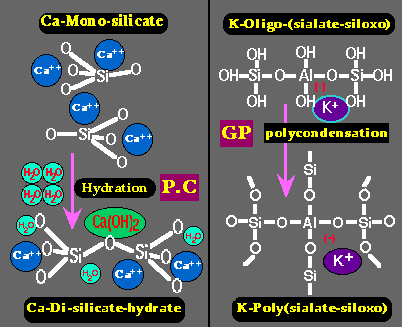
Left: hardening of Portland cement (P.C.) through simple hydration of Calcium Silicate into Calcium Di-Silicate hydrate and lime Ca(OH)2.
Right: hardening (setting) of Geopolymer resin (GP) through poly-condensation of Potassium Oligo-(sialate-siloxo) into Potassium Poly(sialate-siloxo) cross linked network.
The Australian Geopolymer Alliance outlines on his web site the following statement:
(…) Davidovits developed the notion of a geopolymer (a Si/Al inorganic polymer) to better explain these chemical processes and the resultant material properties.
(…) To do so required a major shift in perspective, away from the classical crystalline hydration chemistry of conventional cement chemistry
(…) To date this shift has not been well accepted by practitioners in the field of alkali activated cements who still tend to explain such reaction chemistry in portland cement terminology.
In his recent keynote lecture held at the Geopolymer Camp 2012 State of Geopolymer R&D 2012, Prof. J. Davidovits stated that the present situation, which is prevailing among cement scientists, is a major obstacle to any breakthrough and innovation in geopolymer cement/concrete technology and is responsible for the slow implementation of any world-wide industrialization. See also in the LIBRARY the paper #21 Geopolymer cement review 2013.


