Are Pyramids Made Out of Concrete?
Pyramids (1) Are Pyramids Made Out of Concrete?
Pyramids (2) The evidences
Pyramids (3) The formula, the invention of stone
Pyramids (4) Videos and book
Pyramids (5) FAQ for artificial stone supporters
Pyramids (6) Deep misleading publications by geologists
Why do geologists see nothing?
This is due to the geological glue, which, though artificial, is seen by the geologists either as an impurity, and therefore useless to study, or as a natural binder. At best, the analysis tools and the working methods of geologists consider the glue as a perfectly natural “micritic binder”. A geologist not informed of geopolymer chemistry will assert with good faith that the stones are natural.
The scientific background, including analysis, formula, stone making, are disclosed in the recently updated book by Prof. Joseph Davidovits Geopolymer Chemistry & Applications, in several chapters, i.e. Chapters 5, 11, 13, 17 and 20.
If you want to know how the knowledge evolved after the Pyramids click on Colosses of Memnon
The chemical formula:
People think that because we use chemicals, it is very easy to find these ingredients in the final product. This is wrong. Thanks to the geopolymer chemistry, the chemical reaction generates natural elements, minerals that can be analysed as natural if scientists are not aware of their artificial nature.
So far, we identified at least two chemical systems, one used for the manufacture of the core blocks (the greatest quantity of stones), the second for the casings. In the recent study by Barsoum, Gangly and Hug , the core blocks are illustrated by the samples MENK, whereas the casings relate to the LAUER and OC samples.
The MENK sample is representative of the blocks forming the core of the pyramids. It consists of numulitic shells, as for the other pyramids. It was taken in a large block belonging to a satellite pyramid of Mykerinos (the one in the middle):
M. Barsoum sent an e-mail to J. Davidovits in June 2004, asking if he had an explanation with respect to their analyzes, in particular the presence of magnesium and the absence of sodium carbonate (natron). How to get magnesium Mg involved in the geopolymeric chemical reaction? But, there was another chemical element just as important as the others in this MENK sample. It is the presence of halite salt, NaCl (cooking salt), as can be seen in the figure made with the SEM / EDS electron microscopical investigation which gives the chemical structure of the geopolymer glue, located between the numulitic fossil shells. The chemical formula of the microconstituent (mc ’) includes a molecule of NaCl.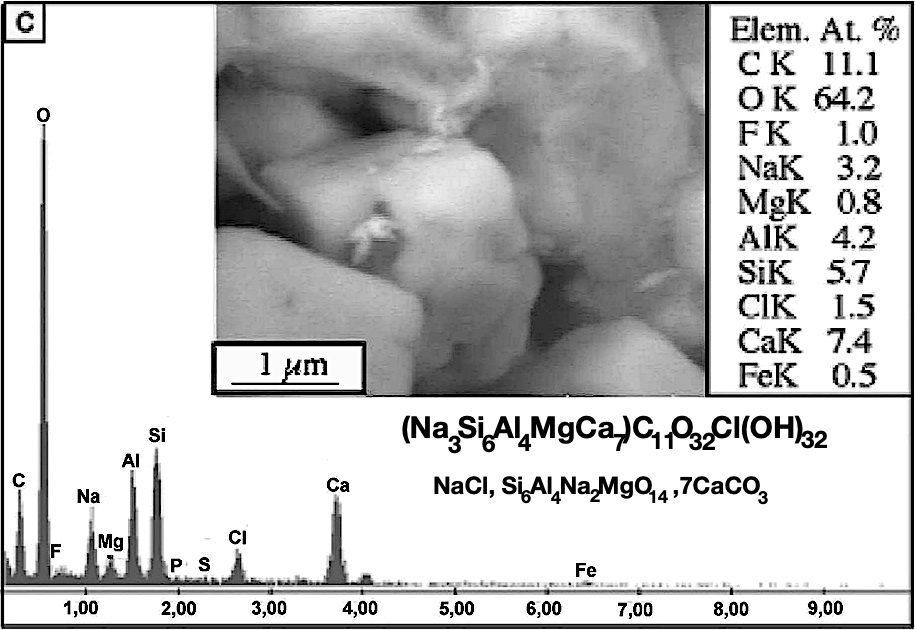
Why is it meaningful? When Davidovits went to the Giza site in 1984, he picked up a few small pieces of stone from the Cheops pyramid. Because he is a chemist, he tasted these stones with his tongue: they were salty. There was NaCl salt in them, cooking salt. Then he took a piece of geological limestone, tasted it: it was not salty. He repeated the experience on each of his visits, in 1988, 1991, 2003; he shared it with his wife Doris and his son Ralph, who accompanied him and confirmed the issue. He pointed this out to the Head of the Chemistry Department at the Palais de la Découverte in Paris, who was preparing an exhibition on the subject titled: How to build a pyramid, and scheduled for Nov. 2006 to April 2007. The chemist was surprised and replied: “It turns out that my daughter is currently on the Giza site; I will send her a message. ” A week later, his daughter confirmed this strange phenomenon to him.
The presence of NaCl salt is just an anecdote for Egyptology. For some Egyptologists, this occurrence of NaCl would be normal since the limestones are sedimented at the bottom of the salty oceans. This is silly reasoning. According to them, all the walls of our buildings and all our cathedrals built with limestone should be covered with salt. They are not, of course. Others say that tourists who urinate on the stones in the rooms leave their mark. Just as silly. Nevertheless, it was present on the stones of all the chambers of the pyramids. Davidovits, in 1988, detached a piece of this salt from the surface of a block located at the top of the corbelled “mortuary” chamber of the Meidum pyramid. But the most significant is the description made by Caliph Al Mamun when he opened in 820 AD the Great Pyramid, which had been sealed for several centuries. He found in the interior rooms that the stone was covered with a layer of 1.5 cm thick cooking salt, halite NaCl.
The blocks of the pyramids do contain halite, NaCl. Since they were made like geopolymer concrete, they also contain moisture. It migrates to the surface, dries, and the NaCl salt crystallizes. We expected to find a migration of sodium carbonate (excess natron) or baking soda, resulting from the chemical reaction of the excess of NaOH alkali with the carbon dioxide in the air. Instead, we are dealing with halite salt, NaCl. Where does it come from? What is the geopolymer chemical reaction generating this NaCl cooking salt?
1) Chemistry of the core blocks
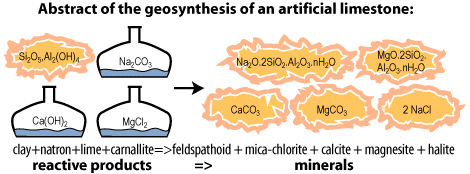
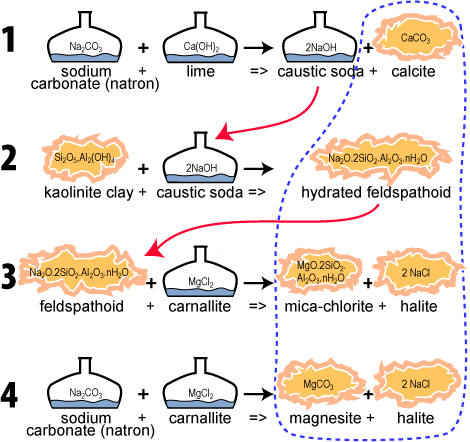
During geosynthesis kaolinite clay (naturally included in the Giza limestone) first reacts with caustic soda (see chemical formula 2). To manufacture this caustic soda, one uses Egyptian natron (sodium carbonate) and lime (coming from plant ashes) (see chemical formula 1). The so obtained caustic soda, NaOH, will react with clay.
The most interesting point is that this chemical reaction creates also pure limestone (calcite) as well as hydrosodalite (a mineral of the feldspathoids or zeolites family).
But, the mixture is still quite caustic. In order to neutralize it, one adds a special salt called carnallite (magnesium chloride) easily found in evaporites, in saline deposits like natron but not at the same place (see chemical formula 3 and 4). Alkalis have been transformed into neutral salt halite, which explains the high content of NaCl found in pyramid stones. Naissant calcite and magnesite may combine to form rhombohedral crystals of dolomite.
2) Chemistry of the casing stones
In the chemical formula 2, the clay may be replaced partially (or entirely) by hydrous siliceous mineral varieties, such as diatomaceous earth (hydrated amorphous) yielding sodium silicate (water glass), which will react with carnallite according to chemical formula 3, and formation of magnesium silicate.
The re-agglomerated stone binders are the result of these geosynthesis (geopolymers) that create several natural minerals: limestone (calcite), hydrated feldspars (feldspathoid, mica-chlorite), magnesium silicates, magnesite (also dolomite resulting from magnesite+calcite) and halite. Egyptian natron often contains Na-sulfate that yields the formation of Ca-sulfate. We understand why geologists can easily be misled.

Imhotep’s formula to make limestone blocks
Imhotep had two different chemical formulas: a very simple one for the casting of the limestone core blocks, and another one to produce the high quality stones of the exterior layer. When all the blocks of the core were set in place, a layer of casing was applied. This meant preparing a more sophisticated type of mold to produce inclined limestone blocks following the slope of the pyramid, adding new ingredients to the mixture to yield a higher quality stone.
1. SOFT LIMESTONE

Pouring Natron salt to the reaction basin
To build the Step Pyramid, Imhotep located a quarry of soft limestone, just one kilometer from the construction site to provide the raw material he needed to cast millions of modular stones. Soft limestone can be easily disaggregated either under pressure or by diluting it in water.
Shallow canals were dug in the soft limestone along the Nile, forming ideal basins for producing large quantities of muddy limestone. Imhotep’s men began disaggregating the clayish soft rock with its water, until the lime and the clay separated, forming a mud with the fossil shells at the bottom.
2. NATRON SALT

The mixing of lime, Natron, limestone and water.
Next, a substance called Natron salt (sodium carbonate) was poured in. Salt is a very reactive substance that has a petrifying effect, which is why it is used to avoid the putrefaction of organic tissue (mummification).
Natron is found in very great quantities in the desert and in Wadi-El-Natron.
3. LIME

The limestone concrete paste.
More lime, the mineral which binds, was added. Lime is a powdery residue obtained by burning and reducing to ashes sedimentary rocks such as limestone and dolomite. The fire oxidizes and converts the rocks into a powdery residue, and that is lime. The ashes of plants are also rich in lime and the priests established the custom of receiving ashes from cooking fires from all over Egypt, to add them to the mixture.
4. CAUSTIC SODA

The first mold filled with limestone
Lime mixed with natron and water produced a third substance, a much more corrosive one, that sparks off a strong chemical reaction and transforms other materials. The water dissolved the Natron salt and put the lime in suspension, forming caustic soda.
Caustic Soda is the catalyst Imhotep needed to trigger off a powerful chemical reaction, one which would produce the fast integration of silica and alumina.
5. CEMENT

The leveling of the second mold.
Men mixed the ingredients in the canals until a homogeneous binder paste was obtained. Imhotep had invented a water-based cement. Now, he had only to convert that cement into concrete.
6. LIMESTONE CONCRETE
His workers added more fossil shells, limestone rubble and silt from the river Nile, producing a concrete paste, which they carried up to where hundreds of small wooden molds had been prepared. These molds had been smeared with rancid oil to facilitate the release of the concrete once hardened.
The mixture was rammed into the molds as in the making of the packed earth called pisé, becoming a dense re-agglomerated limestone, which was let to dry in the shade, to avoid its cracking under the glare of the hot sun.
7. LIMESTONE BLOCKS

The filling of the third mold.
The hardened blocks were released from their molds and easily carried up to the construction site, by means of small ramps over the tiers already set, until the men placed each block in its correct place.
The towering Step Pyramid was not only the first, but also the only one made entirely of small modular blocks weighing approximately 60 kilos apiece, easily carried by two men.
8. IMPROVING THE MANUFACTURE
 The twelve-tonne limestone blocks.
The twelve-tonne limestone blocks.
This Step Pyramid was just the first one that simply took the crude brick techniques and, instead of using mud, Imhotep used a limestone paste. Then, the three Sneferu’s pyramids improved step by step the technology by increasing the size of the blocks and the height of the monuments. Whereas the pyramids at Giza showed how technical improvements helped to achieve one of the famous wonders of the world, only 60 years after the first pyramid at Saqqarah. In later times, by Sneferu’s Red Pyramid, at Dashour, much heavier blocks were molded and cast directly on the spot, which means they were not moved. This is how the Great Pyramids at Giza were built.
At the Geopolymer Institute, we tried to replicate this masterpiece by making life size blocks, that is to say from 1 to 4.5 tonnes. The next pages illustrate our experience.
RECIPE USED IN THE NEXT PAGE VIDEO
1) Nummulitic limestone outcrop (fossil shells), naturally friable, from Tracy-le-Val south of Saint-Quentin (France). It resembles that of Giza but does not contain kaolinitic clay, which has to be added.
2) Into the pool containing two cubic meters of water are poured 160 kg of kaolinitic clay to imitate the Giza limestone, followed by 60 kg of sodium carbonate (natron) and 80 kg of slaked lime.
3) The geological glue is mixed with 4500 kg of limestone using a simple wooden paddle.
After drying, the final mixture contained between 18-20% weight water.
An example of a re-agglomerated limestone
How the pyramid blocks were built ?

Does the picture show an artificial or a natural stone? Scientists of the Geopolymer Institute have successfully manufactured and cast a re-agglomerated limestone. The geological material used here is very similar to the one found at the Giza plateau in Egypt, a soft material with lots of nummulitic shells coming from a quarry in France. The purpose of this test was to demonstrate that this type of limestone is perfect for re-agglomeration. We have disaggregated this soft material with water, then mixed the muddy limestone and its fossil shells with kaolin clay, and a simple geopolymeric binder. Then, the limestone mud was packed into the mould (a pyramid shape!). This re-agglomerated limestone, bonded by a geochemical reaction, thus hardened into a resistant block, much harder than the original material. We have strengthened the stone and made it more resistant to pollution, acid rain, and freezing.

A close-up of the mini-pyramid. Fossil shells are intact and the geopolymer binder is integrated within the calcite matrix.
The mini-pyramid is 9 cm (3.55 inch) large. In these pictures, you can clearly see that anyone who is not aware of the possibility of the geopolymer chemistry can be easily fooled. The final result has not a modern concrete appearance. It is a natural limestone, the material was not crushed but gently disaggregated, and all fossil shells are intact.

A close-up of the bottom of the mini-pyramid. The bottom was the top of the mould, the mini-pyramid was cast upside-down.
Because we were not authorized to sample original materials from the Giza plateau quarries, we did not used the exact ancient Egyptian formula. The French limestone, used in this experience, is very similar but has no reactive clay in it, and we had to add some. Nevertheless, the final result is chemically and geologically close to what we find in Egypt.
With the Egyptian formula, the result is different because it requires bigger blocks for a better cohesion. It is not suitable for small items. Whatever the formula, we have clearly demonstrated that the key of success is an appropriate raw material.


